| |
|
|
 |
|
 |
Fused Silica
Fused Silica (also named Fused Quartz, Fused SiO2 ) is the glassy form of quartz and is thus
isotropic. It is tough and hard and has a very low expansion. Normal
varieties contain water that gives strong absorption in the IR.
Water-free varieties are available.
Vitreous silica is the
generic term used to describe all types of silica glass, with
producers referring to the material as either Fused Quartz or as
Fused Silica. Originally, those terms were used to distinguish
between transparent and opaque grades of the material. Fused Quartz
products were those produced from quartz crystal into transparent
ware, and Fused Silica described products manufactured from send
into opaque ware.
Today, however, advances in raw material
beneficiation permit transparent fusions from sand as well as from
crystal. Consequently, if naturally occurring crystalline silica
(sand or rock) is melted, the material is simply called Fused
Quartz. If the silicon dioxide is synthetically derived, however,
the material is referred to as synthetic Fused Silica.
These
materials are ultra pure, single component glasses (SiO2) with a
unique combination of thermal, optical and mechanical properties,
which make them the preferred materials for use in a variety of
processes and applications where other materials are not suitable.
The very high purity (over 99.9%) ensures minimum contamination in
process applications.
These materials can routinely withstand
temperatures of over 1250ˇăC, and due to their very low coefficient
of thermal expansion can be rapidly heated and cooled with virtually
no risk of breakage due to thermal shock.
These materials are
inert to most substances, including virtually all acids, allowing
their use in arduous and hostile environments.
The dielectric
properties and very high electrical receptivity of these materials
over a wide range of temperatures, together with their low thermal
conductivity allow their use as an electrical and thermal insulating
material in a range of environments.
Fused Silica (Fused
Quartz) is less expensive vitreous silica formed by fusing naturally
occurring quartz crystal or lower grade synthetic stock material,
The UV use is limited to 250nm and this material is usually used for
windows covering visible wavelengths.
Fused Silica is
vitreous silica formed by fusing high purity synthetic material. The
UV use can be reached about 160nm.
Ultraviolet Grade Fused
Silica: JGS1 (Chinese), equivalent to Suprasil 1 and 2 (Heraeus),
Spectrosil A and B (Saint-Gobain) and Corning 7940 (Corning),
Dynasil 1100 and 4100 (Dynasil).
Optical Grade Fused Silica :
JGS2 (Chinese), equivalent to Homosil 1, 2 & 3 (Heraeus),
Dynasil 1000 & 4000 and 5000 & 6000 (Dynasil)
IR grade
Fused Silica: JGS3 (Chinese), equivalent to Suprasil 300
(Heraeus).
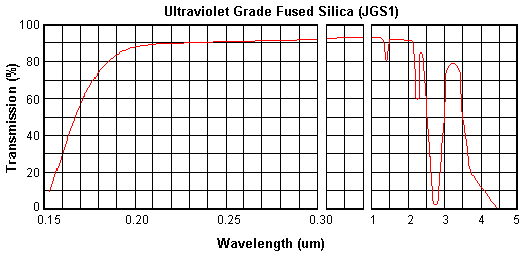
UV grade Fused Silica (JGS1) is synthetic
amorphous silicon dioxide of extremely high purity. This
non-crystalline, colorless silica glass combines a very low thermal
expansion coefficient with good optical qualities, and excellent
transmittance in the ultraviolet. Transmission and homogeneity
exceed those of crystalline quartz without the problems of
orientation and temperature instability inherent in the crystalline
form. Fused silica is used for both transmissive and reflective
optics, especially where high laser damage threshold is
required.
JGS1 is transparent in the ultraviolet and visible
regions, and has no absorption bands in the 170-250 nm wavelength
intervals. It has an intensive OH absorption band in the interval of
wavelength 2600-2800 nm.
JGS1 is used for optics operating
in the deep UV and the visible wavelength range (Laser Lenses,
Windows, Prisms, Mirrors, etc.). It is practically free of bubbles
and inclusions.
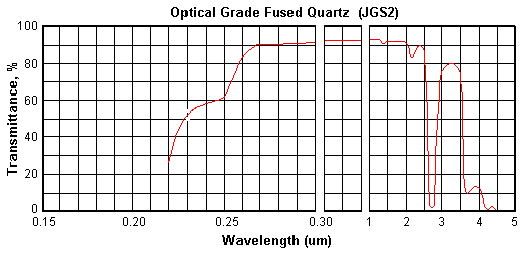
Optical Grade Fused Quartz (JGS2) provides
good UV and visible transmission. It has almost the same physical
and chemical properties with JGS1. However only in thin & small
sheet pieces, JGS2 is virtually bubble-free. Elements built from
larger pieces will most likely contain bubbles, so application
should not be sensitive to these inclusions. But in cases where
simple light gathering and strong mechanical properties are the
primary goals, JGS2 grade provides excellent performance at a low
price.
Ideal Applications for JGS2:
ˇńCondenser optics not concerned with scatter or
distortion
ˇńHigh temperature and
pressure applications
ˇńOptical flats,
microscope slides and sight glasses
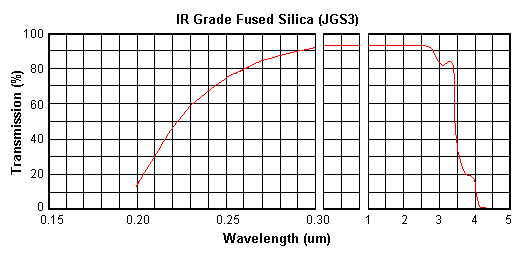
IR grade Fused Silica (JGS3) is super purity synthetic fused silica manufactured by
melting of super pure ash in vacuum. It is transparent in the
ultraviolet, visible and infrared spectral regions. It has no
absorption bands in the visible region and has no OH absorption band
at 2700 nm ("water band").
JGS3 combines excellent physical
properties with outstanding optical characteristics in the deep UV
and the IR wavelength range. It is the preferred material for
transmission optics; it is usually used in IR applications and also
in those requiring very wide wavelength range from DUV to MIR.
However the most common Fused Silica for infrared use is quite a bit
more expensive than Silicon and slightly less expensive than Calcium
Fluoride or ZnS Multi-spectral grade. Also a commercial quality of
IR quartz often contains many small bubbles and should only be used
for non-imaging applications.
OP-Unite can handle with JGS1
well into 10-5 S/D and lambda/20 grade.
Main Properties
Difference properties |
Parameter|Value |
JGS1 |
JGS2 |
JGS3 |
Maximum Size |
<¦µ200mm |
<¦µ300mm |
<¦µ200mm |
Transmission Range
(Medium transmission
ratio) |
0.17~2.10um
(Tavg>90%) |
0.26~2.10um
(Tavg>85%) |
0.25~3.50um
(Tavg>85%) |
OH- Content |
1200 ppm |
150 ppm |
5 ppm |
Fluorescence (ex 254nm) |
Virtually Free |
Strong v-b |
Strong V-B |
Impurity Content |
5 ppm |
20-40 ppm |
40-50 ppm |
Birefringence Constant |
2-4 nm/cm |
4-6 nm/cm |
4-10 nm/cm |
Melting Method |
Synthetic CVD |
Oxy-hydrogen melting |
Electrical melting |
Applications |
Laser substrate: Window, lens, prism,
mirror... |
Semiconductor and high temperature
window |
IR substrate |
Same Properties |
Density |
2.20g/cm3 |
Abbe Constant |
67.6 |
Refractive Index (nd) at 588nm |
1.4586 |
Wavelength (um) |
Refractive Index (n) |
Wavelength (um) |
Refractive Index (n) |
0.200 |
1.55051 |
1.000 |
1.45042 |
0.220 |
1.52845 |
1.064 |
1.44962 |
0.250 |
1.50745 |
1.100 |
1.44920 |
0.300 |
1.48779 |
1.200 |
1.44805 |
0.320 |
1.48274 |
1.300 |
1.44692 |
0.360 |
1.47529 |
1.500 |
1.4462 |
0.400 |
1.47012 |
1.600 |
1.44342 |
0.450 |
1.46557 |
1.700 |
1.44217 |
0.488 |
1.46302 |
1.800 |
1.44087 |
0.500 |
1.46233 |
1.900 |
1.43951 |
0.550 |
1.46008 |
2.000 |
1.43809 |
0.588 |
1.45860 |
2.200 |
1.43501 |
0.600 |
1.45804 |
2.400 |
1.43163 |
0.633 |
1.45702 |
2.600 |
1.42789 |
0.650 |
1.45653 |
2.800 |
1.42377 |
0.700 |
1.45529 |
3.000 |
1.41925 |
0.750 |
1.45424 |
3.200 |
1.41427 |
0.800 |
1.45332 |
3.370 |
1.40990 |
0.850 |
1.45250 |
3.507 |
1.40566 |
0.900 |
1.45175 |
3.707 |
1.39936 |
Transmission Curve |
See below |
|
|
|
Hardness |
5.5 - 6.5 Mohs' Scale 570 KHN 100 |
Design Tensile Strength |
4.8x107 Pa (N/mm2) (7000 psi) |
Design Compressive Strength |
Greater than 1.1x109 Pa (160,000 psi) |
Bulk Modulus |
3.7x1010 Pa (5.3x106 psi) |
Rigidity Modulus |
3.1x1010 Pa (4.5x106 psi) |
Young's Modulus |
7.2x1010 Pa (10.5x106 psi) |
Poisson's Ratio |
0.17 |
Coefficient of Thermal Expansion |
5.5x10-7cm/cm.ˇăC (20ˇăC-320ˇăC) |
Thermal Conductivity |
1.4 W/m.ˇăC |
Specific Heat |
670 J/kg.ˇăC |
Softening Point |
1683ˇăC |
Annealing Point |
1215ˇăC |
Strain Point |
1120ˇăC |
Electrical Receptivity |
7x107 ohm.cm (350ˇăC) |
Dielectric Properties (20ˇăC and 1
MHz)
Constant
Strength
Loss Factor
Dissipation
Factor |
3.75
5x107 V/m
Less than 4x10-4
Less
than 1x10-4 |
Velocity of Sound-Shear Wave |
3.75x103 m/s |
Velocity of Sound/Compression Wave |
5.90x103 m/s |
Sonic Attenuation |
Less than 11 db/m MHz |
Permeability Constants (cm3mm/cm2 sec cm of
Hg)
Helium
Hydrogen
Deuterium
Neon |
(700ˇăC)
210x10-10
21x10-10
17x10-10
9.5x10-17 |
Chemical Stability (except hydrofluoric) |
High resistance to water and
acids |
|
|
|
|
|